PRACTICAL pH Workbook
What is pH?
pH is the measurement of acidity or alkalinity in a substance. It is expressed as a number from 0 to 14. Mathematically, it is defined as:
pH = -log (H+) or more correctly
pH = -log (H30+)
the negative logarithm of the hydrogen ion activity in an aqueous solution
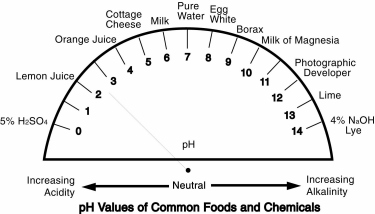
But what does that mean? pH gives us a way of identifying whether a substance is acidic or basic. The numbers from pH 0 to pH 7 represent substances that are acidic, the numbers from pH 7 to pH 14 indicate basic or alkaline materials and pH 7 is neutral (neither acidic nor basic). Figure 1(page 3) gives examples for pH values of various substances.
The relative strengths of various materials being measured increase as the values move away from pH 7. For example, battery acid (which is sulfuric acid, pH 0) is a very different solution from lemon juice, which is also an acid (citric acid, pH 2).
Let's take a quick look at the mathematical definition. One key word here is "log." In the real world, this means that each unit change in pH represents a tenfold change in the strength of the material being measured. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4. A solution that is pH 3 is 100 times more acidic than one at pH 5. Conversely, a solution with a pH of 11 is ten times more alkaline (basic) than a solution with a pH of 10. Interestingly, if one considers that pH covers the range of 0 to 14, that means it represents a measurement that has a span of 0 to 1 X 10'", which is an incredibly large range of measurement.
Why Is pH Important?
The measurement and control of pH is not found only in the laboratory. There are many industries where pH control is necessary. Below are a few examples of some practical areas where pH is important. Food chemists have discovered that jelly will not gel above a pH of 3.5 or below a pH of 2.5. pH regulation has changed jelly making from a haphazard art to a profitable industry. Soil chemists have now determined optimum growth pH ranges for most commercial crops. By closely monitoring and regulating soil pH, farmers have significantly increased their yield per acre. Biochemists have established 7.3 to 7.5 as the critical pH range for human blood. Since disease can alter the body's pH regulation ability, the monitoring of blood pH is an important function of the clinical laboratory. Wine chemists have also found the measurement of pH a valuable aid in wine making. The pH is closely followed in the grape before harvest, as well as during malo-lactic fermentation and the stabilization process after harvest. The making of a fine wine is still an art, but it now has a much more certain outcome. With growing concerns about food safety, pH is becoming an important test method to ensure safe foods. Harmful bacteria and fungus can be controlled and/or eliminated through the careful monitoring and control of pH.

How is pH measured?
COLOR METHODS
Over the years, researchers have discovered many dyes and chemicals that will change color at prescribed pH values. Litmus paper is a good example of a commonly used indicator. In an alkaline solution, the paper turns blue and in acid solution, the paper will turn pink. pH indicator papers of today are accurate to within 0.3 pH units. These indicator strips use multiple color bands to aid in the proper reading of pH. There are two major drawbacks however to the use of indicators. First, the color change is difficult to detect in highly colored or turbid solutions and second, chemical interference with the indicator can invalidate the test. With the invention of the pH probe and meter scientists were able to eliminate these drawbacks as well as increase the precision of pH measurements.
INSTRUMENTAL METHODS
There are four major components of instrument based pH measurement: the measuring electrode, the reference electrode, the pH meter and the buffers. Instrumental pH measurement can be performed relatively fast with a high degree of precision.
THE ELECTRODE
The body of the pH-sensing electrode can be of ordinary glass or plastic construction. However, the electrode culminates in a bulb that is made of a very special glass. When a thin membrane of this glass, which is permeable to hydrogen ions, separates two solutions of varying hydrogen ion concentrations (pH), an electrical potential (voltage) is created at the glass/liquid interface. Any change in the potential of the electrode system will be due to the potential created on the outer surface of the glass membrane. The voltage output changes in proportion to the pH of the sample solution. For ease of handling, the pH sensing electrode and the reference are usually combined into one common body. This one probe design enables users to measure very small samples. There is a new advancement in the measurement of pH. ISFET technology now allows for a solid-state electrode that is glass free and extremely rugged. These new probes are excellent for semi-solids, sauces, thick liquids, and soil samples. Additionally, only a small sample is required for a measurement.
TME METER
The pH meter registers the voltage produced at the electrode. Electronic circuitry within the meter measures voltage changes and converts the value to pH. The voltage is a linear function of pH. At room temperature, 25oC, the meter will register a change of one pH unit for approximately every 59 mV that it measures from the electrode. Therefore, at 25oC pH 7 buffer should read 0 mV and pH 4 should read +177 mV. Any differential reading from 0 mV (actual) in the 7 pH buffer is termed the offset of that electrode. The differential in the actual mV reading vs. theoretical mV reading at pH 4 is called the slope of the electrode. Most meters are now fully digital and microprocessor based. This means that a few push buttons control the meter. In fact, most digital meters offer automatic buffer recognition, (e.g. 4,7, and 10 buffers are automatically recognized). Regular calibration is critical to accurate measurement.
TEMPERATURE COMPENSATION
Due to the physical characteristics of the glass bulb, mV values will differ at temperatures other than 25°C, as defined by the Nernst equation. In order for the meter to correctly measure pH under all conditions, it should be equipped with a control for temperature compensation. The temperature compensation control sets the correct sensitivity of the meter relative to the temperature of the sample to be measured. Initially, this required a separate thermometer and a dial on the meter to correct for the temperature. Most meters now offer a separate temperature probe so that temperature compensation can be automatic. Additionally, there are now many pH electrodes that have the measuring, reference and temperature electrodes in one probe. These probes are very specialized and usually sold only with a corresponding meter that can decode the input properly. In the product selection pages these probes are called 3-in-1 or all-in-1 electrodes.
|
E- Ex
+(2.3RTk/n F)(log(ai)) Nernst Equation Where: |
|
Typical Operation Procedure for pH meter and electrode
After receiving your electrode and meter soak the electrode in a pH buffer 4 overnight. This will activate the electrode. When you are ready to use your system, plug the electrode into the meter rinse the electrode off in distilled water and shake off excess liquid. Take a small sample of pH buffer 7 solution. Check the temperature of the buffer and adjust the meter to that temperature using the temperature adjustment knob. If you have a meter that has automatic temperature compensation and is equipped with a temperature probe, this step is not necessary. Now, immerse the tip of the electrode into the buffer 7. Using the standardize (or calibration) knob, adjust the meter to read 7.00. If you have a microprocessor meter simply depress the key that says 'Stand', 'Cal', or 'Set', and the meter will automatically read the buffer value. Remove the electrode from the buffer rinse and shake off excess liquid. Now it is necessary to set the slope. This can be done with either pH buffer 4 or 10. Use buffer 4 if most measurements are below pH 8, and use pH buffer 10 if most measurements are above pH 8.
Once the correct buffer has been determined, immerse the electrode into the proper buffer and once again adjust the temperature knob if needed. Using the slope control, adjust the meter to the correct value of the buffer being used. Rinse off the electrode, shake off the excess liquid and the meter is now ready to make accurate pH measurements. When taking pH measurements of samples, remember to adjust for temperature, rinse off the electrode in between samples, and shake off excess liquid.
Frequently Asked Questions about pH Measurement
Should I stir my sample? YES
There are two main advantages to slowly stirring or gently agitating the sample during a pH measurement. First, the increased flow of the sample across the electrode results in a faster response time. Second, the solution is properly homogenated so no areas of increased or decreased pH exist.
When is a pH reading stabilized?
It is difficult to know the exact time to take a pH reading. In general, one minute is required to obtain a stable reading. If a reading is taking an exceptionally long time to stabilize, this can indicate several conditions: a clogged electrode junction, a non-homogeneous solution, a troublesome sample with low ionic strength, or a non-aqueous sample.
Is my probe working correctly?
80-90% of all problems with pH measurement result from a faulty electrode or improper calibration. The first step in resolving pH measurement problems is to determine if the probe is working correctly. First, examine the electrode carefully for breaks or cracks in the glass portion. Even a hairline crack renders the electrode useless and the electrode must be discarded.
Next, check the electrode for excessive KCI crystals, either in the bulb or clogging the filling hole. If this is the problem and your electrode is refillable, gently empty the chamber and rinse with de-ionized or distilled water. This should dissolve the clogging crystal. Again, gently empty the chamber and refill with the proper filling solution for your specific electrode. Soak overnight in 1 M KCI before using. For a non-refillable electrode, continue to the next step.
If there are no visible cracks or KCI crystals, a clogged reference junction may be the problem. First, use a dilute dishwashing detergent or isopropyl alcohol. This step will remove any oil or grease deposits that can clog the junction. If this fails to rectify the problem, immerse the electrode tip into 0.1 N HCI for 15 seconds, rinse in tap water. Then immerse tip into 0.1 N NaOH for 15 seconds and rinse in tap water. Repeat this three times. If electrode performance has not been restored, soak the tip of the electrode in concentrated NH4OH (1 M) for 10-15 minutes. Rinse thoroughly with distilled water. Soak for 10-15 minutes in pH 4.0 buffer prior to use. (Caution: NH4OH is very caustic and irritating. All work should be done in a fume hood.) This procedure is for Ag/AgCl electrodes only.
Electrode Care
Many electrode problems can be eliminated with reasonable care. Proper storage, use, and maintenance decreases down time and provides maximum lifetime.
STORAGE
1. Electrode should be stored in an acidic solution with a low salt content. Commercial soaking solution5 are available; see the product section at the end of this book.
2. Long term storage - If an electrode is to be stored over 2 weeks then both the refillable and gel-filled electrodes should be stored in storage solution.
USE
1. Electrodes should always be used in a vertical position.
2. Electrodes should be rinsed between samples with distilled or deionized water. NEVER wipe an electrode to remove excess water; just blot the end of the electrode with a lint-free paper. Wiping an electrode can cause spurious readings due to static charges or may scratch the bulb surface which can permanently damage the electrode.
3. The level of filling solution in refillable electrodes should be kept at least 2/3/3 full. The filling hole should be open during use.
4. pH electrodes are fragile. A proper electrode holder should be used to provide support and aid in raising and lowering the probe into solutions.
Electrode Selection Chart
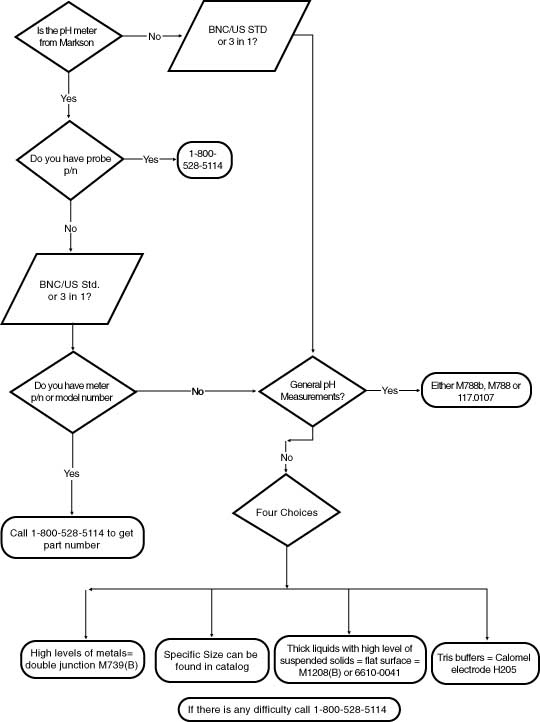
What type of connectors is available on electrodes?
The most common connectors on electrodes are the BNC connector or the US Standard connector. Both connectors are pictured below. Choose the connector that is compatible with the meter you are using. Most pH electrodes are equipped with a 3-foot cable. Optional 5, 10, or 20' extension cables are also available. BNC Connector U.S. Standard Connector
Troubleshooting Guide For Three Common pH Problems
PROBLEM 1
Meter exhibits no response when measuring pH.
Action
• Check power to meter.
• Meter not plugged in.
• Dead batteries in battery-powered meter.
• Electrode not properly connected.
CONCLUSION:
If the meter and electrode
seem to be connected correctly, proceed to the next step. Check millivolt
function of the pH meter.
• Turn selection control to mV (millivolt). Place electrode in fresh pH 7.0
buffer at 25C. Meter should read 0 mV +/-15 mV.
• Place electrode in fresh pH 4.0 buffer at 25oC. Meter should read +177 mV
+/-15 mV.
• Place electrode in fresh pH 10.0 buffer at 25C. Meter should read -177 mV
+/-15 mV.
CONCLUSION:
• If pH meter responds correctly in mV mode, this pinpoints the fault to the pH
board. The pH meter needs to be serviced. Call us at 1-800-528-5114 for service
information. If the pH meter does not respond, you may have a faulty pH meter
or faulty electrode. Proceed to next step.
Check circuitry of the pH
meter.
• Turn selection control to mV or pH.
• Open paper clip to U shape or use a piece of wire. Short the input to the
meter by touching one end of paper clip to the measuring input and the other to
the reference input. In a BNC style (most common) one shorts the inside of the
input with the outside of the input. Note: This procedure does not pose any
electrical risk if strictly followed.
• This should result in a stable needle reading of OVM or pH 7, which can be
deflected approximately 2 pH units using the offset, set or standardize
control.
• If the pH meter responds
correctly when shorted, the meter is in good working order and the problem is
probably a faulty electrode. Go to the next step.
• If the pH meter does not respond correctly when shorted, the meter is faulty
and requires repair. Markson LabSales has a fully equipped repair department.
Call us at 1-800-528-5114 for more information. Check for a faulty electrode. ·
If a faulty electrode is suspected, replace with a new or other working
electrode and recheck pH function. · If another electrode is unavailable, a new
electrode should be ordered. Markson LabSales offers a full line of pH
electrodes. Call us at 1-800-528-5114 for a copy of our free catalog.
PROBLEM 2
Unable to calibrate meter
Action
• Check temperature control
to verify correct setting.
• Open a new bottle or make a fresh batch of standard buffer and recheck
calibration.
• Check electrode for physical defects. Visually check electrode for cracks or
other abnormalities. A cracked or damaged electrode should be replaced.
CONCLUSION:
If no defects are seen, go to next step. Clean the electrode to eliminate a clogged reference junction. This procedure is for Ag/AgCl electrodes only. Immerse the tip of the electrode into concentrated NH4OH for 10-15 minutes. Caution: NH4OH is very caustic and should be used in a hood. Rinse the electrode. Soak in pH 4.0 buffer for 10-15 minutes. Recheck calibration.
CONCLUSION:
If cleaning does not result in improved performance, the meter could be at fault. Contact Customer Service at 1-800-528-5114. Troubleshooting Guide For Three Common pH Problems (continued)
PROBLEM 3
pH readings are unstable, slow, erratic, or drift.
Action
• Cheek the sample. ·A
changing sample temperature. Allow sufficient time for a sample temperature to
stabilize. Note: Vigorous stirring on an uninsulated stirring motor can lead to
small but significant sample temperature changes.
• A non-uniform sample. Gentle stirring using an insulated stirring motor can
eliminate pH "zones" which result in erratic or drifting readings.
• A sample that is incompatible with the pH electrode. When measuring pH of
special solutions such as HF, strong oxidizing solutions, or solutions that contain
elements that can poison an electrode, be certain that you are using the
correct electrode.
If you have questions. Call us at 1-800-528-5114 and our technical service group will help.